FALSE
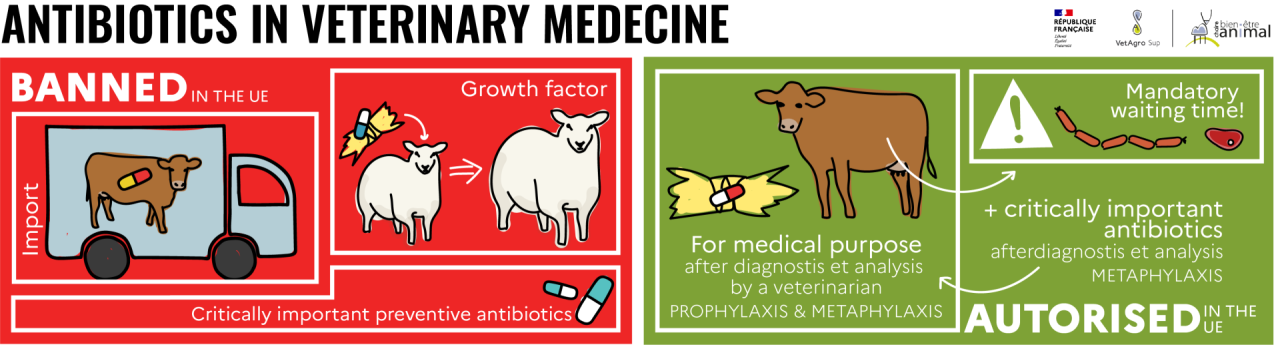
Antibiotics are strictly forbidden in animal feed as growth promoters in the European Union. In very rare cases, after diagnosis and prescription by a veterinarian, and to facilitate administration to a group of animals, feed containing antibiotics may be used for medical purposes.
Keep in mind
- Bacteria are everywhere
- Antibiotics are essential for combating certain infectious diseases caused by pathogenic bacteria and for maintaining the health and welfare of animals.
- Some bacteria develop resistance to one or more antibiotics.
- Antibiotic resistance is a major public health problem, causing many people to die every year.
- Antibiotics must therefore be used rationally (in veterinary and human medicine) to limit the phenomenon of antibiotic resistance.
- Antibiotic use in farm animals has fallen sharply since 2011, both in general and in feed.
Basics: bacteria, antibiotics, human and veterinary medicine
Different kinds of infectious agents cause disease and infection in humans and animals. These can be parasites, fungi, viruses or bacteria.
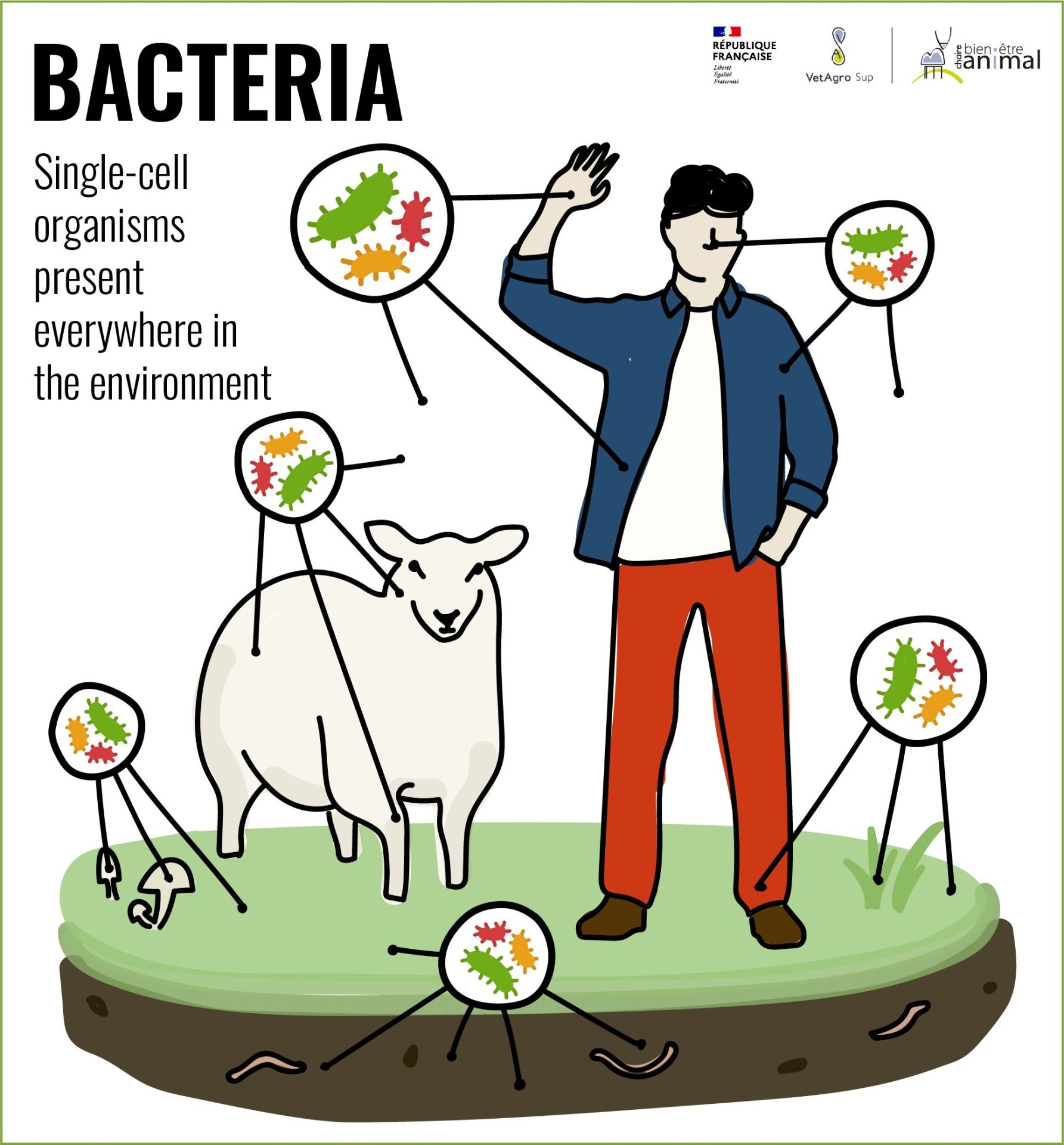
Bacteria?
Bacteria are single-celled organisms found everywhere in the environment. They represent around 13% of total biomass, while animals account for only 0.5%[1].
In humans, as in animals, they form an important part of the microbiota and are found not only in the digestive tract, but also on skin, in the eyes and in various cavities (nose, ear, vagina, etc.).
Under normal circumstances, the bacteria in the microbiota are not pathogenic and do not cause disease. However, if the immune system is weakened or if the microbiota is unbalanced, notably through misuse of antibiotics, certain bacteria in the microbiota can act as pathogens.
Did you know?
Every individual, human or animal, has his or her own microbiota, which gradually builds up from birth. This microbiota plays a decisive role in an individual's state of health. The intestinal microbiota, for example, is involved in digestion and the functioning of the immune system. The microbiota is made up of bacteria, but also of other microorganisms such as viruses.
Other bacteria are not normally present in the body and have greater pathogenic power. Under certain conditions, they can cause infectious disease when they develop in humans or animals.
Certain pathogenic bacteria can be transmitted from animals to humans (or vice-versa) and cause zoonoses, i.e. infectious diseases transmitted from animals to humans through direct or indirect contact. Well-known zoonoses include tuberculosis[2],and salmonellosis[3].
Antibiotics?
Antibiotics are substances with the power to inhibit or even eliminate the growth of bacteria. They can be produced by microorganisms or through chemical synthesis.
Antibiotics are used in human and veterinary medicine to treat infectious diseases caused by bacteria, after the appearance of clinical signs. However, antibiotics are not effective against infectious diseases caused by microorganisms other than bacteria (such as viruses or single-cell eukaryotic[4] parasites).
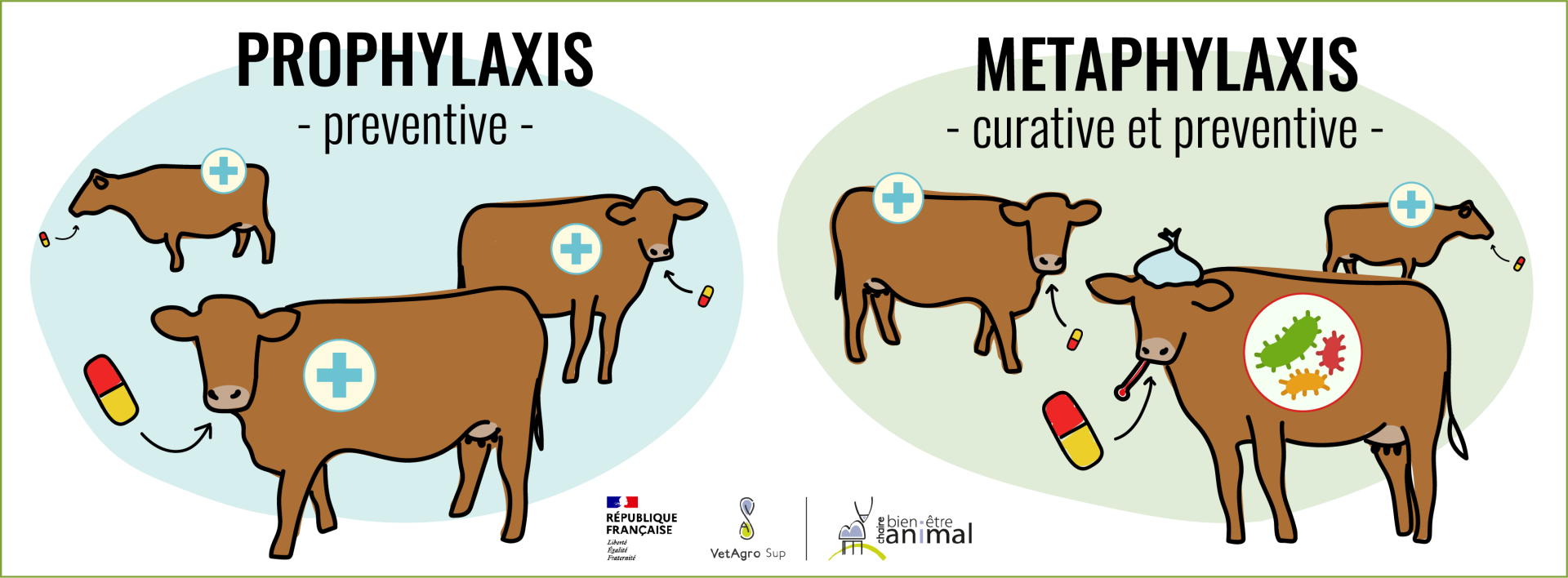
They can be administered to infected individuals, or those at risk of becoming infected but not yet ill, to prevent the spread or aggravation of the disease in other individuals. This is known as “prophylaxis” – which refers to the preventive use of a drug before the onset of the clinical symptoms of a disease with the aim of preventing its outbreak – or “metaphylaxis” – which is a mix of curative treatment for clinically ill individuals and preventive treatment to stop the spread of the disease to individuals in close contact with sick individuals.
Properly used, antibiotics keep people and animals healthy. What’s more, the toxicity of antibiotics is relatively low, which explains why they have been so widely used since their discovery – the so-called “antibiotic miracle“.

Did you know?
Antibiotics were first "discovered" in 1877 by Pasteur and in 1897 by French physician Ernest Duschene. However, it is Alexander Fleming, an English biochemist, who is regarded as the father of antibiotics with his accidental discovery of penicillin in 1928.
It is important to remember, however, that antibiotics are not the only treatments available to combat infectious diseases. Vaccination and preventive measures, such as biosecurity measures[5]for example, are highly effective and ultimately reduce the use of antibiotics while improving animal health and welfare.
Antibiotics and antibiotic resistance
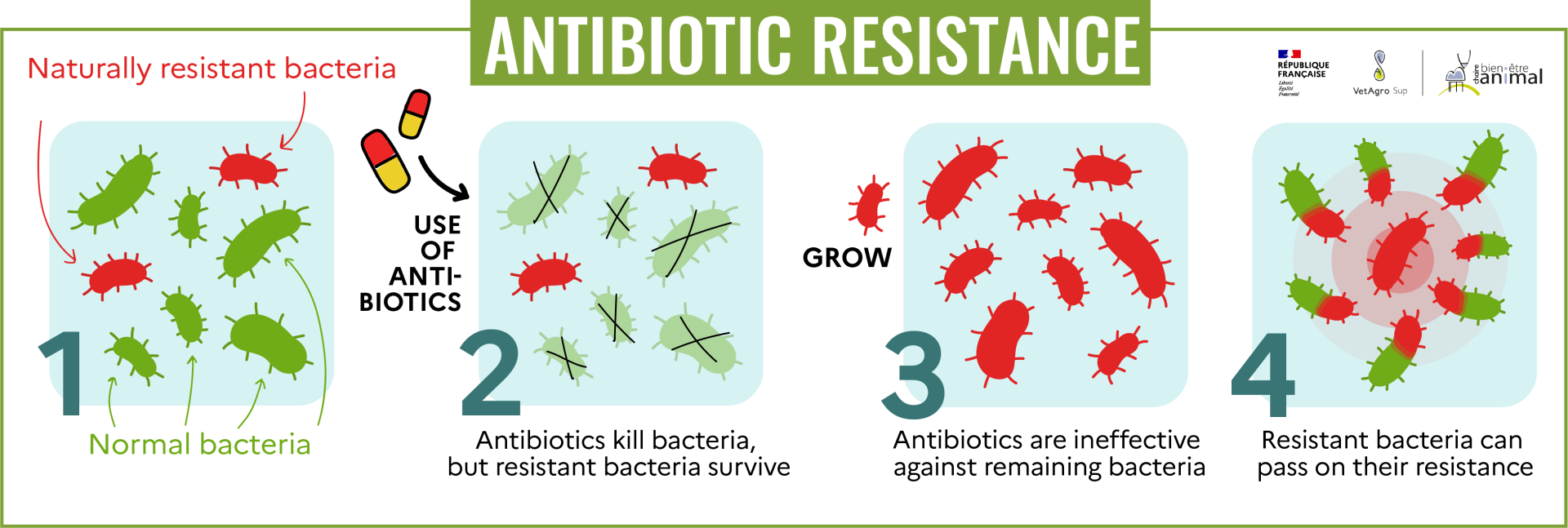
Bacteria have a very high rate of random genetic mutation (and they reproduce very rapidly, which further favors the appearance of mutations). Certain mutations can develop defense mechanisms in bacteria, enabling them to resist antibiotics. Moreover, bacteria are constantly exchanging genetic material with one another, and can therefore transfer genetic material that supports resistance, spreading it rapidly. This is known as antibiotic resistance.
📌 For more information
Historically, these resistant bacteria have been rare. However, when antibiotics are used in human or veterinary medicine, non-resistant bacteria are eliminated, leaving only resistant bacteria to survive (and develop) and then enjoy “more room”. . Incorrect use of antibiotics (wrong prescription, treatments that are too short or too long, or inappropriate dosages) encourages this mechanism[6].
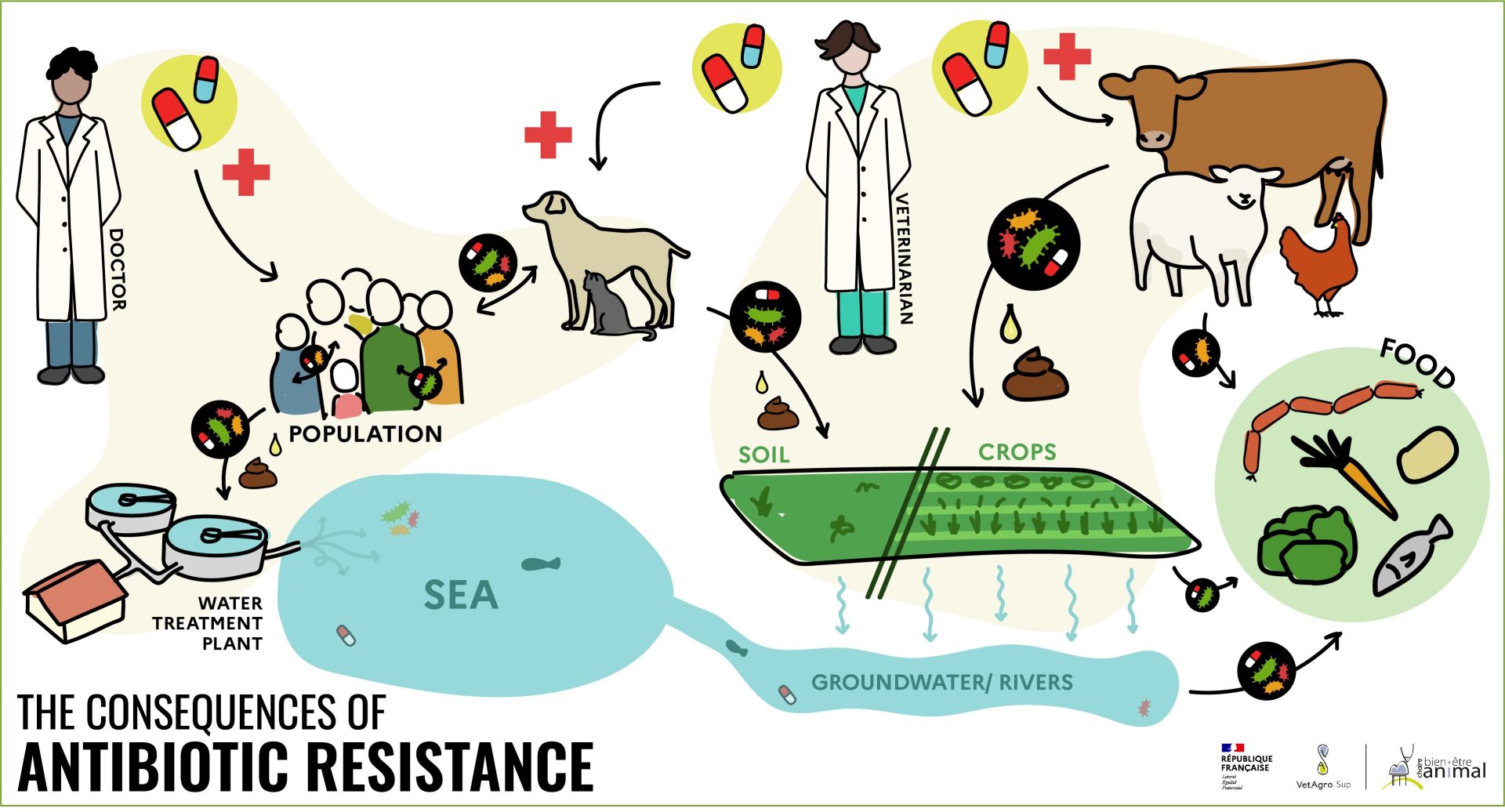
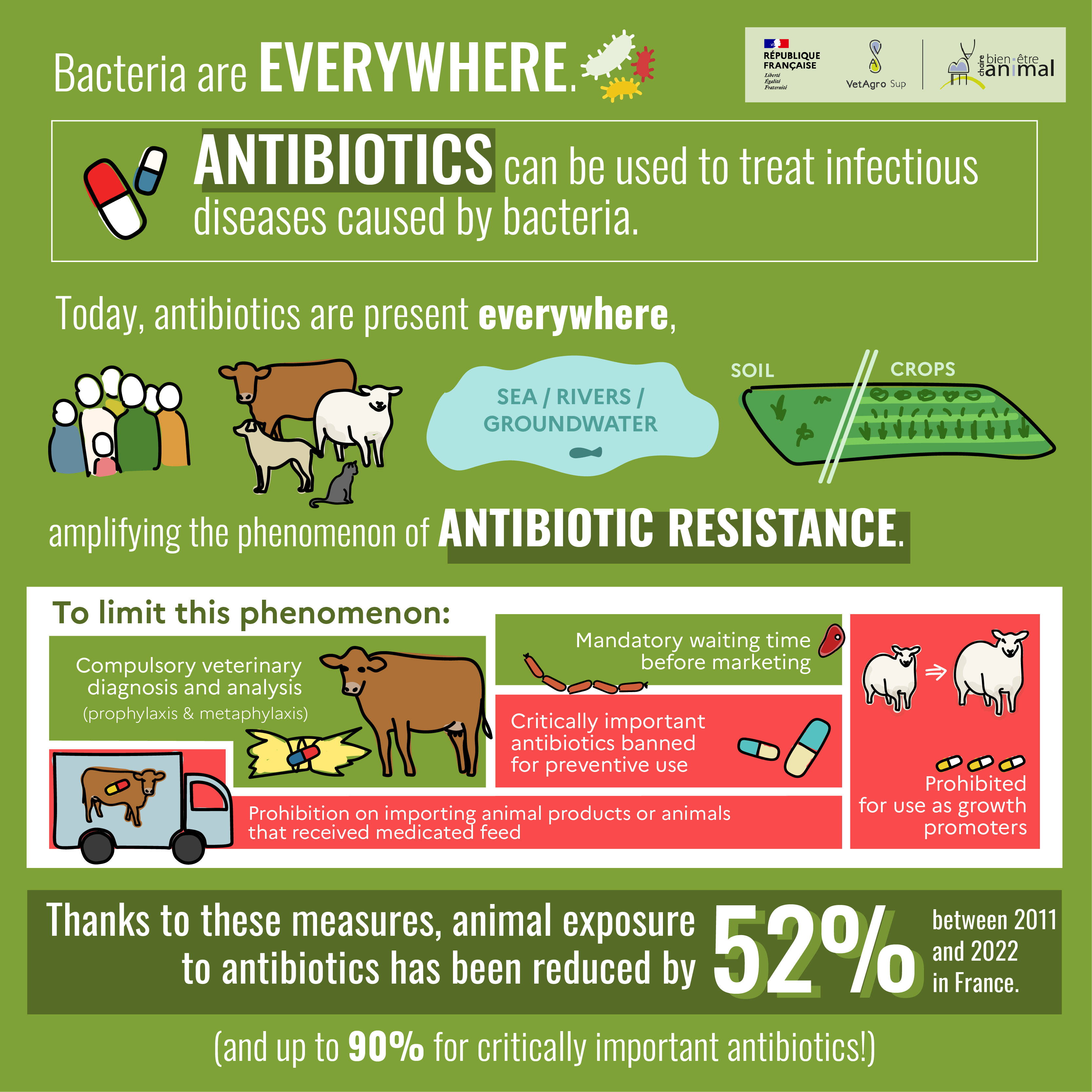
This mechanism of antibiotic resistance can occur with an individual’s own bacteria. What’s more, humans and animals release some of the antibiotics they have absorbed, through feces or wastewater, which can then foster the appearance of resistant bacteria in the environment[7] [8]. Also, some environments – such as hospitals – have a very high antibiotic pressure, which eliminates non-resistant bacteria and favors resistant bacteria. These days, antibiotics are everywhere, amplifying the phenomenon of antibiotic resistance.
These resistant bacteria can then spread through direct contact (between humans, between animals or between animals and humans) or indirect contact (discharge of resistant bacteria in wastewater, for example, or through animal feces, which can contaminate food). As a result, resistant bacteria can be found in waterways downstream of towns and farms, and even in groundwater[9].
Antibiotic resistance renders ineffective some or even most of the antibiotics needed to treat certain infections in human medicine. An estimated 5,500 people die every year because of antibiotic resistance in France[10].
Antibiotic resistance is therefore considered by the World Health Organization (WHO) to be one of the major global threats to public health. The WHO has published a list of bacteria against which new antibiotics are urgently needed, in order to treat them and counter resistance[11].

In brief
Appropriate, limited use of antibiotics in both human and veterinary medicine is essential to prevent the emergence or development of resistant bacteria.
Proper use of antibiotics in veterinary medicine
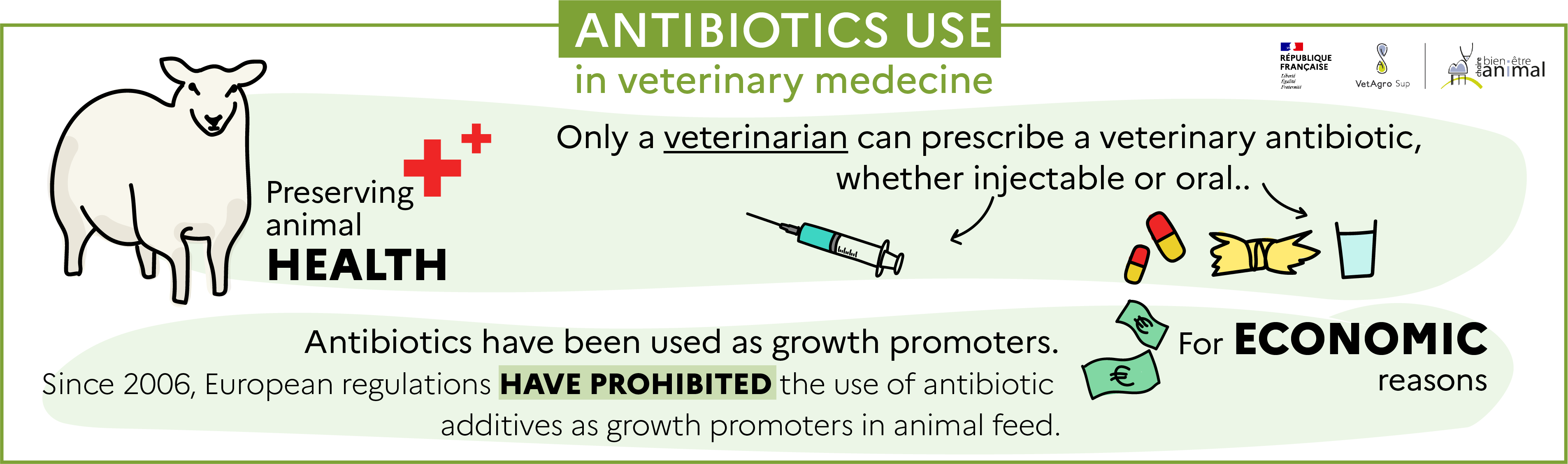
Antibiotics are necessary to combat certain bacterial infections that can be common in farm animals, and can lead to a significant deterioration in their health and thus their welfare, or even to their death. However, to limit the emergence of resistant bacteria, the use of antibiotics is regulated in veterinary medicine. Indeed, only a veterinarian can prescribe a veterinary antibiotic, and good practices are recommended in the decree of July 22, 2015[12].
In animals, antibiotics can be administered by injection (intravenous, intramuscular, subcutaneous or intramammary), or orally (as tablets or in feed or drinking water).
Historically, antibiotics have also been used in production animals as growth promoters. . . Incorporated into feed at low doses and then distributed in the form of medicated feed, they modified the intestinal microbiota and led to better assimilation of feed, resulting in improved animal performance (growth, for instance). They were then used for economic purposes, rather than to preserve animal health.
Antibiotics and antibiotic resistance
Since 2006, European regulation no. 1831/2003[13] prohibits the use of antibiotic additives as growth promoters in animal feed. Animals are therefore not fed medicated feed containing antibiotics. Since January 2022, European regulation 2019/4[14] also prohibits the import of animal products or animals that received medicated feed.
When animals are ill, however, the use of feed containing antibiotics may be authorized to facilitate their administration to a group of animals, but this is subject to strict controls. This can only be done for medical, prophylactic or metaphylactic purposes, following a diagnosis and a benefit-risk analysis carried out by a veterinarian, and not on a systematic basis, nor as growth promoters.
The use of certain critically important antibiotics
Certain antibiotics are considered to be of critical importance[15] in human medicine. In fact, they are the only treatments available for certain serious human diseases. They are therefore reserved for human medicine in order to limit their use and limit the risk of antibiotic resistance developing. . Their conditions of use are regulated by decree no. 2016-317[16] . In animals, they cannot be used preventively and may only be prescribed after a veterinary diagnosis followed by an antibiogram[17] in a curative or metaphylactic context.
Withdrawal period after antibiotic use in livestock
When an animal is treated with antibiotics, there is a regulatory waiting time[18] before any product (milk or meat from cattle, for example) can be marketed. In fact, the presence of antibiotics in a food product may present a toxicological or allergic risk for the consumer, or prevent the product from being properly processed (e.g. cheese) or even encourage the emergence of antibiotic resistance. This withdrawal time is determined for each drug so that the level of active substances from the drug is sufficiently low in the food to be considered harmless for consumers[19].
In addition to this mandatory waiting time, antibiotic residue checks are carried out very regularly in slaughterhouses to avoid their inadvertent presence in animal products intended for human consumption[20]. In fact, the risk of antibiotic residues being present in food destined for human consumption is virtually non-existent..
The evolution of antibiotic use in animals
Trends in overall antibiotic use
To limit the risk of antibiotic resistance and promote good practices, the French Ministry of Agriculture and Food Sovereignty has, since 2011, set up three successive national plans, the Ecoantibio 1 (2012-2017), Ecoantibio 2 (2017-2022) and finally Ecoantibio 3 (2023-2028) plans aimed at limiting the use of antibiotics in livestock farming.
The Ecoantibio 1 plan called for a 25% reduction in the use of antibiotics in veterinary medicine (50% for critically important antibiotics)[21]. The Ecoantibio 2 plan did not include a quantified overall reduction target[22]However, it did set a reduction target for Colistin, a critically important antibiotic, and aimed to consolidate the results obtained[23].
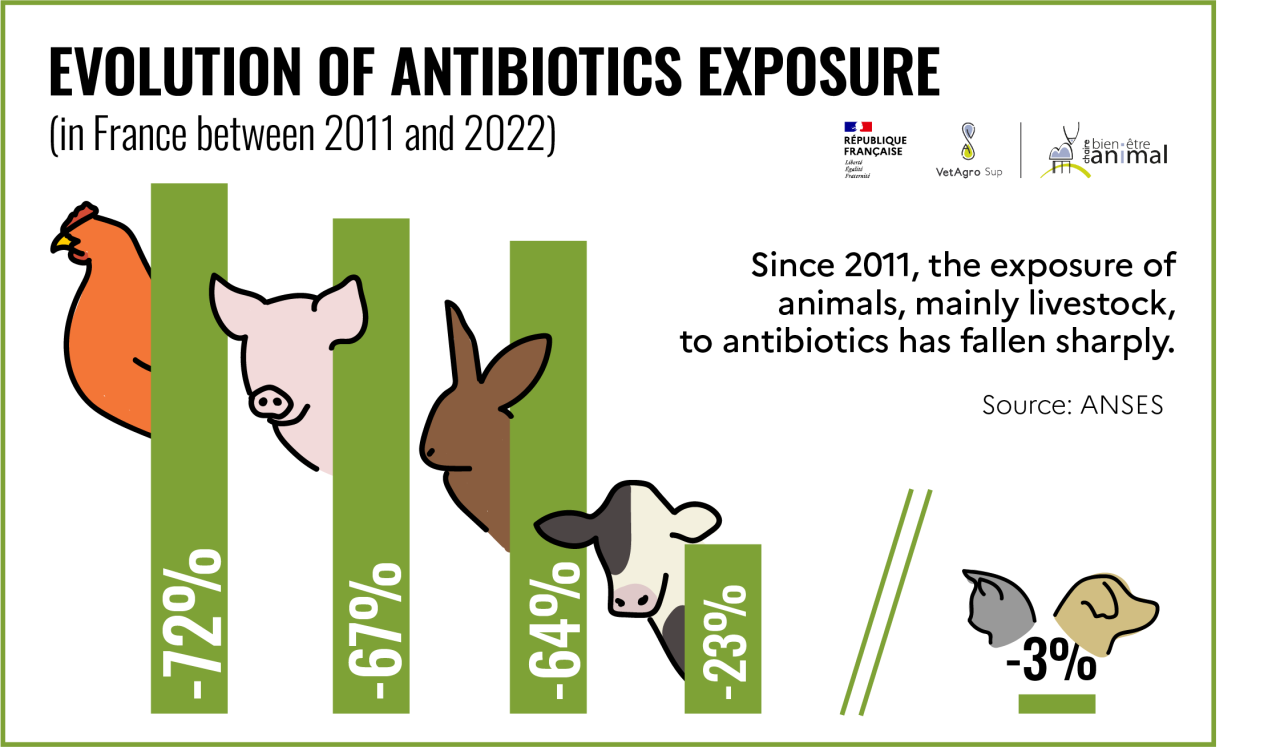
According to data published by ANSES[24], entre 2011 et 2022, de diminuer en France l’exposition des animaux aux antibiotiquesthe involvement of the various stakeholders (veterinarians, farmers, pharmacists, scientists, public authorities), between 2011 and 2022, has made it possible to reduce the exposure of animals to antibiotics[25] in France by 52%, and antibiotics of critical importance to human health by 90%[26].
This decrease has affected all livestock species, with antibiotic exposure falling by 72% for poultry, 67% for pigs, 64% for rabbits and 23% for cattle since 2011. This reduction is less marked for companion animals (3% for dogs and cats)[27]. The aim of the Ecoantibio 3 plan is therefore to maintain low levels of exposure for farm animals and to reduce the level of exposure for pets by 15%.
Total antibiotics sold in animal health will be 70% lower in 2022 than in 2011 – the reference year of the first Ecoantibio plan – and critically important antibiotics will account for only 0.3% of total[28].
Evolution of antibiotics in animal feed
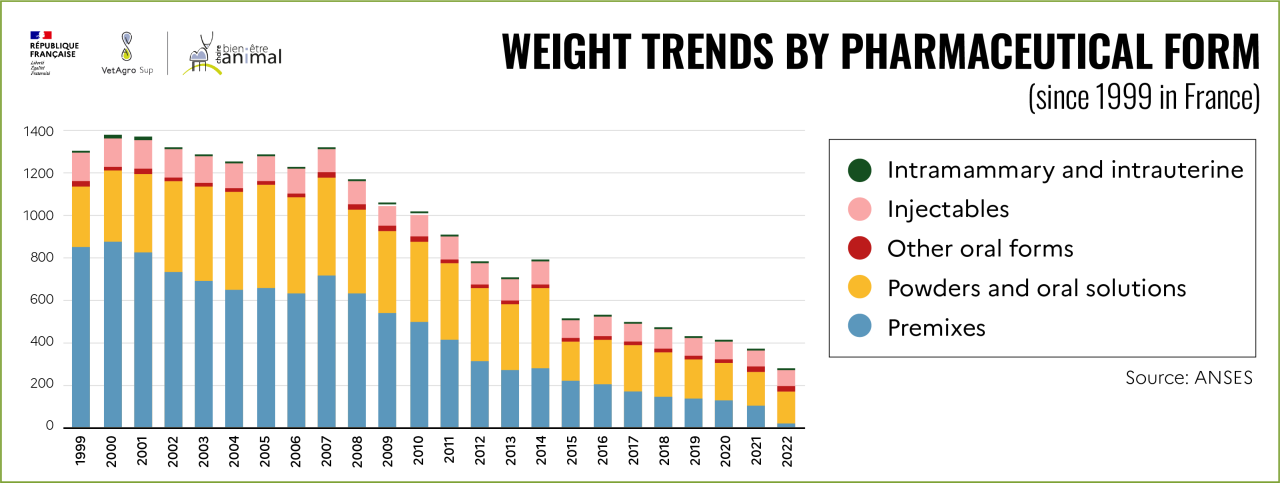
Changes in European regulations have led to a drastic reduction, or even elimination, in the use of antibiotics in animal feed. Antibiotics sold in medicated feed in France have fallen from around 400 tons in 2011 to 21 tons in 2022, a 95% reduction. This decrease is particularly sharp for 2021 and 2022[29].
In 2022, of the 21 tons of antibiotics still sold via feed, 32% will be used for pigs, 23% for sheep, 16% for rabbits and 15% for poultry. According to data published by ANSES, this corresponds to just over 56,000 tons of animals[30] treated with medicated feed, i.e. less than 1% of all meat consumed by the French population[31].
This reduction in the use of antibiotics in medicated feeds has not been offset by increased use of antibiotics in other forms, as can be seen in the graph below.
Farm animals cannot be fed antibiotics since 2006. A veterinarian may sometimes prescribe antibiotics in feed, to facilitate distribution to animals, but only in a medical context and following a diagnosis. The use of antibiotics in animal health has fallen sharply since 2011. However, efforts still need to be maintained, particularly for companion animals, but also in human medicine. Indeed, the phenomenon of antibiotic resistance concerns everyone, and a “one health” approach involving veterinarians, doctors and ecologists is absolutely essential to preserve human health, animal health and the health of ecosystems.
In short
For further information
Check out this video produced by La Semaine Vétérinaire, which looks at the use of antibiotics in veterinary practice for pets:
Thanks to Roman Mounier, intensive care physician, Georges Pompidou European Hospital, Raphael Guatteo, teacher-researcher in bovine health management at Oniris and Bertrand Ridremont, veterinary surgeon, consultant in animal health and nutrition, for their careful proofreading of the text.
[1]https://planet-vie.ens.fr/thematiques/ecologie/relations-trophiques/la-repartition-de-la-biomasse-sur-terre
[2]https://agriculture.gouv.fr/telecharger/118950
[3]https://agriculture.gouv.fr/telecharger/118929
[4]Micro-organisms consisting of a single cell with a nucleus.
[5]Set of protective measures to prevent the introduction of pathogens, their dispersion within the farm and their spread outside the farm. One measure, for example, might be not to bring in animals from outside the farm, or to test their health status before bringing them in.
[6]https://www.inserm.fr/dossier/resistance-antibiotiques/
[7]https://sante.gouv.fr/prevention-en-sante/les-antibiotiques-des-medicaments-essentiels-a-preserver/des-antibiotiques-a-l-antibioresistance/article/une-seule-sante-l-antibioresistance-concerne-les-hommes-mais-aussi-les-animaux
[*8] https://www.lemonde.fr/sciences/article/2018/12/10/les-usines-d-antibiotiques-indiennes-sont-des-fabriques-d-antibioresistance_5395476_1650684.html
[9]https://www.inserm.fr/dossier/resistance-antibiotiques/
[10]https://sante.gouv.fr/prevention-en-sante/les-antibiotiques-des-medicaments-essentiels-a-preserver/des-antibiotiques-a-l-antibioresistance/article/l-antibioresistance-pourquoi-est-ce-si-grave
[11]https://www.who.int/fr/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
[12]https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000031142007/
[13] https://eur-lex.europa.eu/legal-content/FR/LSU/?uri=CELEX:32003R1831
[14] https://eur-lex.europa.eu/legal-content/FR/TXT/PDF/?uri=CELEX:32019R0004&from=FR
[15] According to the WHO (2003) definition, “A critically important antibiotic is defined as an antibiotic belonging to a family of antibiotics that is the only treatment or one of the only treatments available to treat a serious human disease or an intestinal pathogenic bacterium causing a food-borne disease”.
[16] Laboratory test to check the sensitivity of the bacteria present to different antibiotics, in order to determine which antibiotic to use.
[17]https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000032251629/
[18] Time between last administration of veterinary medicinal product and collection of food
[19] Among other things, the waiting time is calculated according to the Maximum Residue Limit (MRL).
The MRL of an active substance is the maximum level of that substance (and in some cases its metabolites) that can be accepted as legally permitted in or on food of animal origin. These values differ, for the same active substance, according to the animal species or tissue concerned (meat, fat and skin, liver, kidney, milk, eggs, honey). Maximum residue limits are set by a decision of the European Commission based on a scientific opinion issued by the European Medicines Agency.
[20] Analysis of 31700 slaughterhouse samples in 2021: https://agriculture.gouv.fr/plans-de-surveillance-et-de-controle
[21]https://agriculture.gouv.fr/plan-ecoantibio-2012-2017-lutte-contre-lantibioresistance
[22] There was, however, a target for Colistin, with a 50% reduction in exposure in the beef, pork and poultry sectors.
[23]https://agriculture.gouv.fr/le-plan-ecoantibio-2-2017-2021
[24]https://www.anses.fr/fr/content/bilan-antibioresistance-vente-antibiotiques-veterinaire-2023
[25] animal exposure to antibiotics is estimated by taking into account the recommended dosage for each drug, as well as the population of each animal species in France
[26] By way of comparison, antibiotic consumption in human medicine, following the 1st national alert plan on antibiotic use, fell by just 28% between 2000 and 2019, from 194 million boxes to 139 million boxes.
[27] https://agriculture.gouv.fr/infographie-ecoantibio-reduire-lutilisation-des-antibiotiques-veterinaires
[28] https://www.anses.fr/fr/system/files/ANMV-Ra-Antibiotiques2022.pdf
[29] Reduction in tonnage from 98 tonnes in 2021 to 21 tonnes in 2022, i.e. a reduction of 82%.
[30] https://www.anses.fr/fr/system/files/ANMV-Ra-Antibiotiques2022.pdf
[31] 5.7 million tonnes of meat were consumed in France in 2022
Keep in mind
- Bacteria are everywhere.
- Antibiotics are essential for combating certain infectious diseases caused by pathogenic bacteria and for maintaining the health and welfare of animals.
- Some bacteria develop resistance to one or more antibiotics.
- Antibiotic resistance is a major public health problem, causing many people to die every year.
- Antibiotics must therefore be used rationally (in veterinary and human medicine) to limit the phenomenon of antibiotic resistance.
- Antibiotic use in farm animals has fallen sharply since 2011, both in general and in feed.
Key Figure
This is the percentage reduction in the overall use of antibiotics for farm animals since 2011.